Simulation with machine learning models
[ad_1]
Hydrogen, the most abundant element in the universe, is found everywhere from the dust that fills most of space to the cores of stars to many substances on Earth. This would be reason enough to study hydrogen, but its individual atoms are also the simplest elements with only one proton and one electron. For David Ceperley, a professor of physics at the University of Illinois Urbana-Champaign, this made hydrogen a natural starting point for formulating and testing a theory of matter.
Credit: Grainger College of Engineering at the University of Illinois Urbana-Champaign
Hydrogen, the most abundant element in the universe, is found everywhere from the dust that fills most of space to the cores of stars to many substances on Earth. This would be reason enough to study hydrogen, but its individual atoms are also the simplest elements with only one proton and one electron. For David Ceperley, a professor of physics at the University of Illinois Urbana-Champaign, this made hydrogen a natural starting point for formulating and testing a theory of matter.
Ceperley, also a member of the Illinois Center for Quantum Information Science and Technology, used computer simulations to study how hydrogen atoms interact and combine to form different phases of matter such as solids, liquids, and gases. However, a correct understanding of this phenomenon requires quantum mechanics, and quantum mechanical simulations are expensive. To simplify the task, Ceperley and his collaborators developed machine learning techniques that allow quantum mechanics simulations to be carried out with an unprecedented number of atoms. They check in Physical Review Letter that their method discovered a new kind of high-pressure solid hydrogen that previous theory and experiment had missed.
“Machine learning has taught us a lot,” says Ceperley. “We had seen signs of the new behavior in our previous simulations, but we didn’t believe it because we could only house a small number of atoms. With our machine learning model, we can take full advantage of the most accurate methods and see what’s really going on.”
Hydrogen atoms make up a quantum mechanical system, but capturing its full quantum behavior is extremely difficult even on a computer. Advanced techniques such as quantum Monte Carlo (QMC) can feasibly simulate hundreds of atoms, while understanding large-scale phase behavior requires simulating thousands of atoms over long periods of time.
To make QMC more flexible, two former graduate students, Hongwei Niu and Yubo Yang, developed a machine learning model trained with QMC simulations that is capable of holding more atoms than QMC on its own. They then used the model with postdoctoral research associate Scott Jensen to study how the solid phase of hydrogen that forms at very high pressures melts.
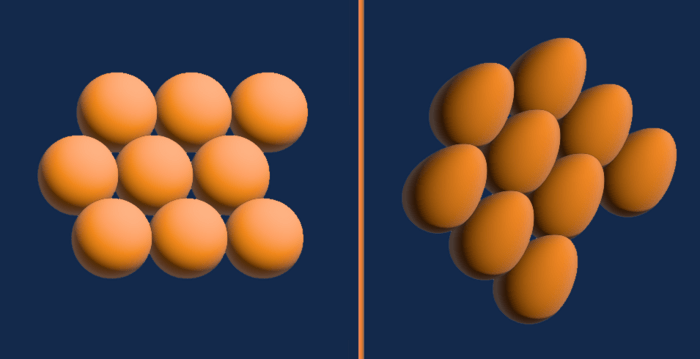
The three of them were surveying different temperatures and pressures to form a complete picture when they noticed something unusual in the solid phase. While the molecules in solid hydrogen are usually nearly spherical and form a configuration called solid hexagonal — Ceperley compared this to a pile of oranges — the researchers observed a phase in which the molecules took an oval shape — Ceperley described it as egg-like.
“We started with the not-so-ambitious goal of perfecting a theory about something we knew,” recalls Jensen. “Unfortunately, or perhaps fortunately, it was more interesting than that. There is a new behavior emerging. In fact, it is the predominant behavior at high temperatures and pressures, something for which there is no clue in older theories.”
To verify their results, the researchers trained their machine learning model with data from density function theory, a widely used technique that is less accurate than QMC but can hold more atoms. They found that the simplified machine learning model perfectly reproduced standard theoretical results. The researchers concluded that their large-scale machine learning-assisted QMC simulations can explain effects and make predictions that standard techniques cannot.
This piece has started a conversation between Ceperley’s collaborators and some of the tryrs. Measurement of high-pressure hydrogen is difficult, so experimental results are limited. The new predictions have inspired several groups to revisit the problem and more carefully explore hydrogen’s behavior under extreme conditions.
Ceperley noted that understanding hydrogen under high temperatures and pressures will improve our understanding of Jupiter and Saturn, gas planets made mostly of hydrogen. Jensen adds that hydrogen’s “simplicity” makes it an important substance to study. “We want to understand everything, so we have to start with a system that we can attack,” he said. “Hydrogen is simple, so it’s important to know that we can deal with it.”
#
This work was carried out in collaboration with Markus Holzmann of the Univ. Grenoble Alpes and Carlo Pierleoni of the University of L’Aquila. Ceperley’s research group is supported by the US Department of Energy, Office of Basic Energy Sciences, Computational Materials Science program under the Award DE-SC0020177.
Journal
Physical Review Letter
DOI
10.1103/PhysRevLett.130.076102
Article title
Stable Solid Molecular Hydrogen above 900 K from the Potential of Machine Learning Trained by Quantum Diffusion Monte Carlo
Article Publication Date
17-Feb-2023
[ad_2]
Source link