Watch molecules relax in real time
[ad_1]
(Nanowerk News) Designing the next generation of efficient energy conversion devices to power our electronics and heat our homes requires a detailed understanding of how molecules move and vibrate when undergoing chemical reactions caused by light. Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have now visualized the distortion of chemical bonds in the methane molecule after it absorbs light, loses electrons, and then loosens. Their studies provide insight into how molecules react to light, which could ultimately be useful for developing new methods to control chemical reactions.
Examining how a molecule responds to light on a very fast time scale allows researchers to track how electrons move during chemical reactions. “The big question is how does a molecule dissipate energy without breaking apart,” said Enrico Ridente, physicist at Berkeley Lab and lead author on Science paper report work“Femtosecond symmetry breaking and coherent relaxation of methane cations via x-ray spectroscopy”). This means examining how excess energy is redistributed in molecules that have been excited by light, as electrons and nuclei move while the molecule relaxes to an equilibrium state.
Investigating this fine-scale motion means making observations of processes that occur at timescales faster than a millionth of a second. For decades, researchers have relied on theory to describe how excess energy affects the symmetry of – but not breaks – the bonds of molecules excited by light. This theory predicts how the bond lengths and angles between individual atoms should change as the positions of the electrons shift, and what intermediate structures should be adopted.
Now, using the ultrafast X-ray spectroscopy facility at Berkeley Lab’s Chemical Sciences Division, Ridente and his colleagues observed how the structure of the ionized methane molecule evolves over time.
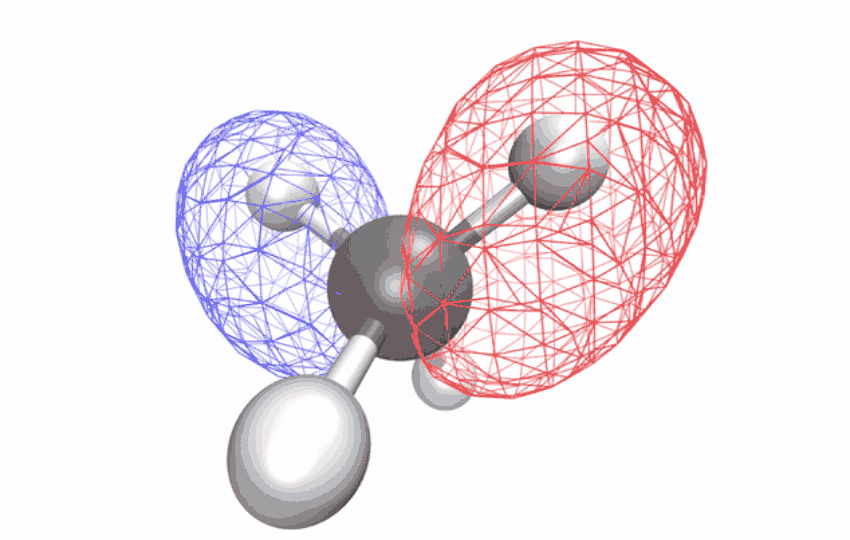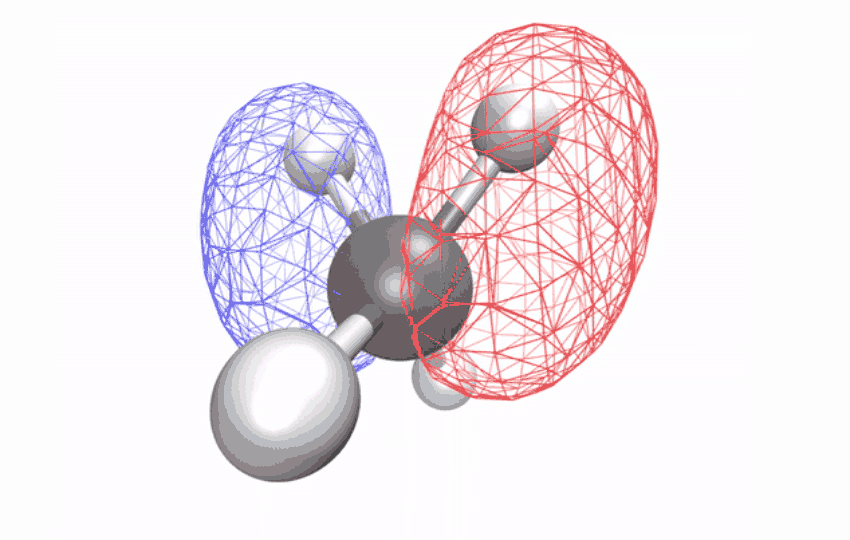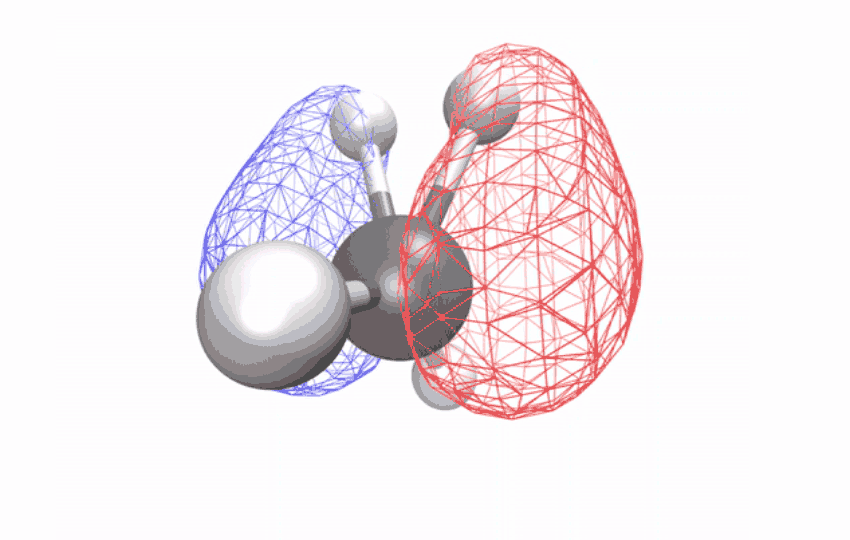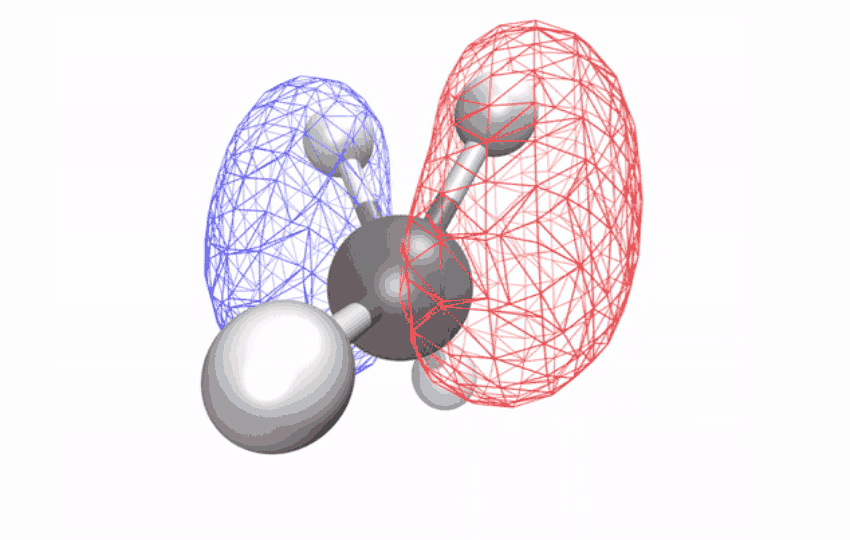
“The methane ion is the ideal system to answer this question because it doesn’t decompose when excited by light,” says Ridente.
By first using a laser to strip electrons from neutral methane molecules, then taking ultrafast X-ray spectroscopic photographs of the remaining ions, the researchers collected time series spectral signals. The signals reveal how an initially symmetrical shape becomes distorted over a period of ten femtoseconds (one femtosecond is one-quarter trillionth of a second) – observational evidence of a long-studied effect called Jahn-Teller distortion. Longer time observations show that for another 58 femtoseconds, the distorted shape vibrates coherently in a scissor-like motion while redistributing its energy through other vibrations through changes in the geometry of the structure.
“Thanks to these measurements and the insights gleaned from theory, we were able to complete the full evolution of distortions for the first time,” said Stephen Leone, chemist at Berkeley Lab and senior author on Science paper.
The researchers used the Cori and Perlmutter system at the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science user facility at Berkeley Lab, to perform calculations that ensure measurements of molecular motion.
“We can now explain how the molecule is distorted after it loses an electron and how the energy of the electron responds to this change,” said Diptarka Hait, a graduate student at Berkeley Lab and lead theoretician of the study.
This study demonstrates the feasibility of an x-ray approach to study ultrafast molecular dynamics. Methane is a fundamental but simple molecule in which one of the most basic types of distortion occurs as predicted, but with a richer and more complicated dynamic than previously understood. “This research opens the door to studying more complex systems and other types of distortion,” said Ridente. Such insights into electron and core dynamics could lead to innovations in new energy conversion devices and photocatalytic applications.
[ad_2]
Source link