Scientists use peroxide to peer into metal oxide reactions
[ad_1]
(Nanowerk News) Researchers at Binghamton University led research in partnership with the Center for Functional Nanomaterials (CFN)—U.S. Department of Energy’s (DOE) Office of Science User Facility at Brookhaven National Laboratory—to better look at how peroxide on the surface of copper oxide promotes hydrogen oxidation but inhibits oxidation carbon monoxide, which allows them to direct oxidation reactions. They were able to observe these rapid changes with two complementary spectroscopy methods that had never before been used in this way.
The results of this work have been published in the journal Proceedings of the National Academy of Sciences (“Tuneing the surface reactivity of oxides with peroxide species”).
“Copper is one of the most studied and relevant surfaces, in both catalysis and corrosion science,” explains Anibal Boscoboinik, materials scientist at CFN. “So many mechanical parts used in industry are made of copper, so trying to understand this element of the corrosion process is very important.”
“I’ve always loved looking at copper systems,” says Ashley Head who is also a materials scientist at CFN. “They have very interesting traits and reactions, some of which are very striking.”
Gaining a better understanding of oxide catalysts gives researchers more control over the chemical reactions they generate, including solutions for clean energy. Copper, for example, can catalytically form and convert methanol into a valuable fuel, so being able to control the amount of oxygen and the number of electrons in copper is a key step toward an efficient chemical reaction.
Peroxide as a Proxy
Peroxide is a chemical compound containing two oxygen atoms connected by a shared electron. The bonds in peroxides are quite weak, allowing other chemicals to change their structure, which is what makes them so reactive. In this experiment, scientists were able to change the redox step of the catalytic oxidation reaction on the surface of oxidized copper (CuO) by identifying the makeup of the peroxide species formed with different gases: O2 (oxygen), H2 (hydrogen), and CO (carbon monoxide).
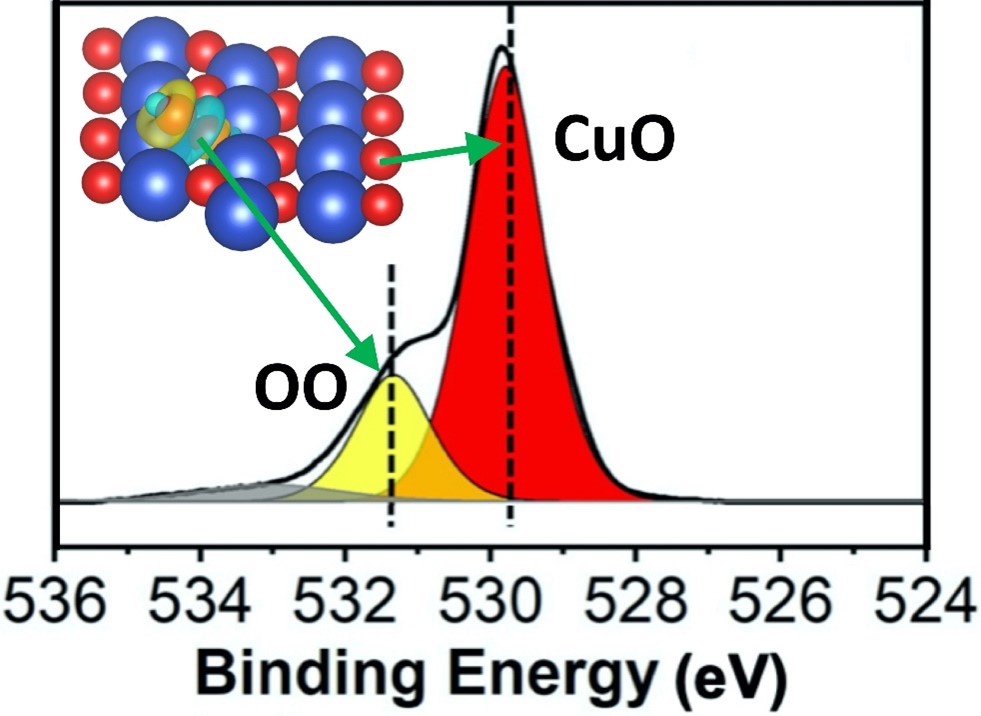
Redox is a combination of reduction and oxidation. In this process, the oxidizing agent gains one electron and the reducing agent loses one electron. When comparing these different peroxide species and how these steps played out, the researchers found that the surface coating of the peroxide significantly increased the reduction of CuO in favor of H2 oxidation. They also found that, conversely, it acts as an inhibitor to suppress the reduction of CuO to CO (carbon monoxide) oxidation. They found that the opposing effect of the peroxide on the two oxidation reactions stems from modifications to the surfaces on which the reactions take place.
By finding these bond sites and studying how they promote or inhibit oxidation, scientists can use these gases to better control how these reactions play out. However, to tune in to this reaction, scientists have to clearly see what’s going on.
The Right Tool for the Job
Study this reaction in place important for the team, because peroxide is very reactive and these changes happen quickly. Without the right tools or environment, it’s difficult to capture such confined moments on the surface.
Peroxide species on copper surfaces have not been observed using in-situ infrared (IR) spectroscopy in the past. With this technique, researchers use infrared radiation to gain a better understanding of the chemical properties of a material by looking at the way radiation is absorbed or reflected under reaction conditions. In this experiment, scientists were able to distinguish “species” of peroxide, by slight variations in the oxygen they carry, which would otherwise be very difficult to identify on the surface of metal oxides.
“I was really excited when I looked at the infrared spectra of these peroxide species on the surface and saw that there weren’t many publications. It was very interesting that we were able to see these differences using techniques that are not widely applied to this kind of species,” recalls Head.
IR spectroscopy alone isn’t enough to tell for sure, which is why the team also used another spectroscopy technique called ambient pressure X-ray photoelectron spectroscopy (XPS). XPS uses low energy x-rays to remove electrons from a sample. The energy of these electrons gives scientists clues about the chemical nature of the atoms in the sample. Having both techniques available through the CFN User Program was key to making this research possible.
“One of the things that we are proud of is the instrument that we have and modified here,” said Boscoboinik. “Our instruments are linked, so users can move samples in a controlled environment between the two techniques and study them in situ for complementary information. In most other circumstances, the user must remove the sample to switch to another instrument, and that change in environment can alter the surface.
“CFN’s good features lie not only in its state-of-the-art facilities for science, but also in the opportunities it provides to train young researchers,” said professor Guangwen Zhou in the Thomas J. Watson College of Engineering and Applied Science’s Department of Mechanical Engineering and Materials Science program at Binghamton University. . “Each student involved benefits from the extensive hands-on experience in the microscopy and spectroscopy tools available at CFN.”
This work was completed with contributions from four PhD students in Zhou’s group: Yaguang Zhu and Jianyu Wang, first co-authors of this paper, and Shyam Patel and Chaoran Li. All of these students are at the start of their careers, having just earned their PhD in 2022.
Future Findings
The results of this study may apply to other types of reactions and other catalysts besides copper. These findings and the processes and techniques that lead scientists there may find their way into related research. Metal oxides are widely used as catalysts themselves or components in catalysts. Tuning peroxide formation on other oxides could be a way to block or enhance surface reactions during other catalytic processes.
“I am involved in several other projects related to copper and copper oxide, including converting carbon dioxide to methanol for use as a clean energy fuel,” said Head. “Seeing this peroxide on the same surface I’m using has the potential to impact other projects using copper and other metal oxides.”
[ad_2]
Source link