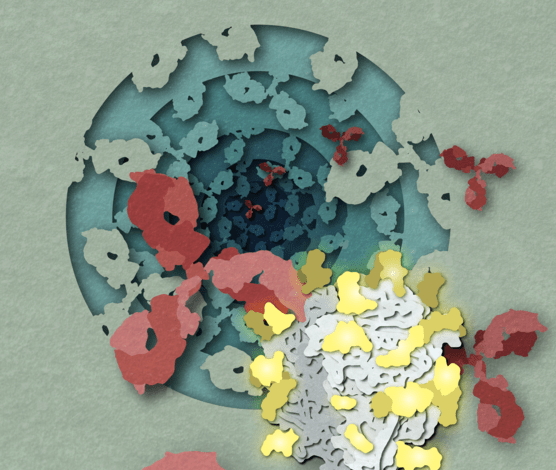
The virulent structure of the virus is leading to new avenues for vaccine design
[ad_1]
LA JOLLA, CA—Every year, hundreds of thousands of people in West Africa are infected with the Lassa virus, which can cause Lassa fever and cause severe illness, long-term side effects, or death. There are currently no widely approved treatments or vaccines for this disease. Now, scientists at Scripps Research have determined the structure of a critical protein complex that allows the Lassa virus to infect human cells. The research, published online at Cell Reportalso identified new antibodies that bind to these proteins and neutralize the virus, paving the way to more effective vaccines and treatments for the Lassa virus.
LA JOLLA, CA—Every year, hundreds of thousands of people in West Africa are infected with the Lassa virus, which can cause Lassa fever and cause severe illness, long-term side effects, or death. There are currently no widely approved treatments or vaccines for this disease. Now, scientists at Scripps Research have determined the structure of a critical protein complex that allows the Lassa virus to infect human cells. The research, published online at Cell Reportalso identified new antibodies that bind to these proteins and neutralize the virus, paving the way to more effective vaccines and treatments for the Lassa virus.
“This work is a major step forward in our ability to isolate novel antibodies to relevant susceptibility sites on the virus, and it provides the basis for conducting rational vaccine designs to protect people broadly from many Lassa virus lineages,” said senior author Andrew. Ward, PhD, professor of Integrative Computational and Structural Biology at Scripps Research. “The new reagents described in this paper are already being put to good use and yielding exciting new results.”
Like many viruses, Lassa virus exists in multiple lineages, each with slight variations in its genes. This diversity makes it difficult to determine antibodies that recognize all versions of the Lassa virus. Scientists have also struggled to isolate the Lassa glycoprotein — the spike-like protein that surrounds viruses and is the target of most antibodies. In infectious viruses, these glycoproteins exist in complexes of three, called trimers. However, for decades, scientists were only able to isolate glycoproteins in the laboratory as a single protein and not in their trimer complex.
In 2022, Ward and colleagues discovered a way to use nanoparticles to aggregate glycoproteins into trimers. In the new work, they used that technique to isolate and characterize the trimer glycoprotein structures of four different Lassa virus lineages. Surprisingly, the structures of the glycoproteins of the different lineages are very similar.
“We hoped to see more distinct differences that would explain why antibodies do not recognize all lineages,” said Hailee Perrett, a Scripps Research graduate student and first author of the work. “In contrast, we found very high levels of conservation across the peptide and sugar components of the protein.”
Using the same stable glycoprotein, Ward, Perrett and their colleagues next used blood samples from patients who had recovered from Lassa virus to isolate antibodies to the trimer glycoprotein. They discovered new antibodies and characterized previously discovered antibodies that recognize different lineages of the Lassa virus glycoprotein, which may be useful in developing treatments or preventive vaccines against the virus.
The team is already planning future experiments to determine more antibodies to the Lassa virus glycoprotein, as well as to further analyze the structure of the protein to identify sites on the glycoprotein that are ideal for drug targeting.
“Our goal is not just to try and determine some of the structural details of these different Lassa viruses, but to provide a basic protocol and resources to the field,” said Perrett. “We hope our approach and initial findings help propel science in this area forward.”
In addition to Ward and Perrett, the authors of the study, “Structural conservation of Lassa virus glycoprotein and recognition with neutralizing antibodies,” include Philip JM Brouwer, Jonathan Hurtado, Grace Gibson, Terrence Messmer, Aleksandar Antanasijević and Bryan Briney of Scripps Research; Maddy L. Newby and Max Crispin of the University of Southampton; Lin Liu and Geert-Jan Boons from the University of Georgia; Helena Müller-Kräuter, Sarah Müller Aguirre and Thomas Strecker from Philipps-University Marburg; Judith A. Burger, Joey H. Bouhuijs and Rogier W. Sanders from the University of Amsterdam; and John S. Schieffelin of Tulane University.
This work was supported by the David C. Fairchild Endowed Fellowship, Achievement Award for Foundations of Higher Education Scientists, National Institutes of Health (1F31Al172358, R01 AI165692, R01 AI171438), Netherlands Scientific Research Organization, amfAR Mathilde Krim Fellowship in Biomedical Research (#110182-69- RKVA), the Vici fellowship of the Netherlands Scientific Research Organization, Fondation Dormeur in Vaduz, Deutsche Forschungsgemeinschaft (197785619/SFB1021), International AIDS Vaccine Initiative (INV008352/OPP1153692) and the Bill and Melinda Gates Foundation (OPP1170236).
Article Publication Date
18-May-2023
[ad_2]
Source link